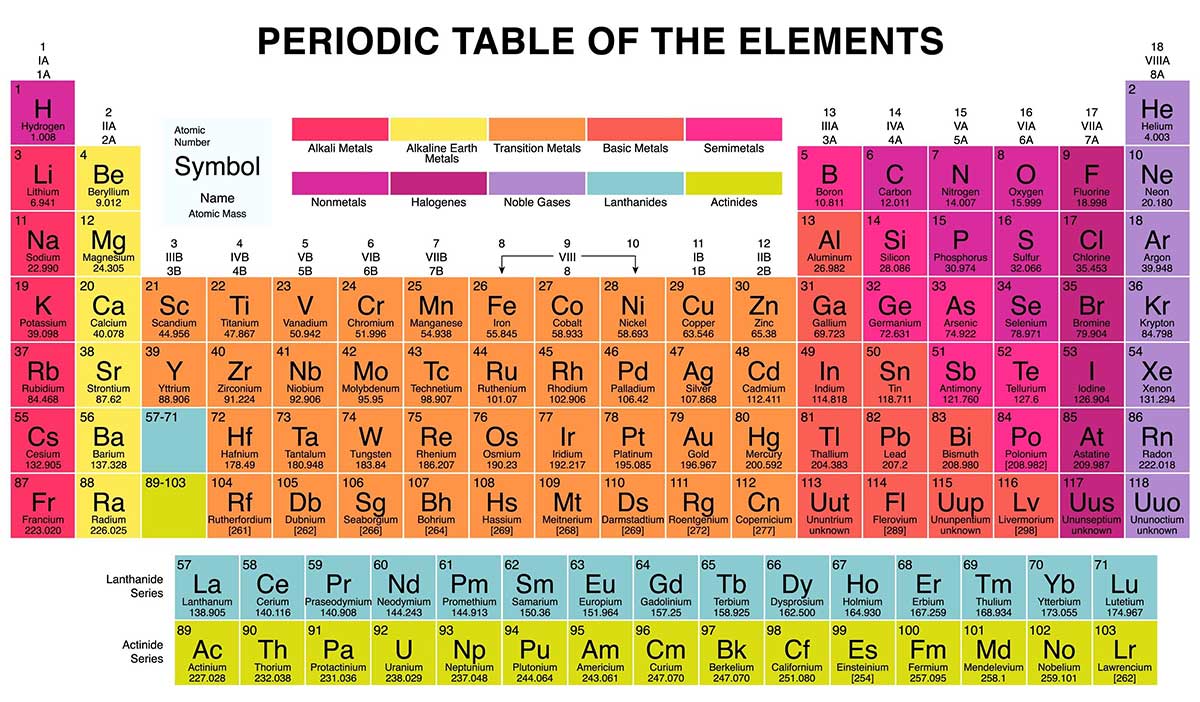
So far, a total of 118 elements are known to us. All the known chemical elements organized in Periodic Table — first published by Russian chemist Dmitri Mendeleev. Elements are placed in Periodic Table in seven rows and 18 columns. Rows are called periods. The row number indicates the number of energy levels required by the electrons of elements in that row to fit in. Columns are called the groups. The column number tells us how many electrons are there in the outermost energy level of element elements in that group. With same number of electrons in the outermost shell the grouped elements behave in similar manner.
There are a total of ten groups in the Periodic Table, namely:
- Group 1: Alkali metals
- Group 2: Alkaline-earth metals
- Group 3: Transition metals
- Group 4: Post-transition metals (aka other metals)
- Group 5: Metalloids (aka semi-metals)
- Group 6: Nonmetals
- Group 7: Halogens
- Group 8: Noble gases (aka inert gases)
- Groups 9: Lanthanides or Lanthanoid (aka rare-earth elements)
- Group 10: Actinides or Actinoids
Groups in Periodic Table
Before we begin, there is a small note about the first element. Hydrogen is the first element in the Periodic Table. A hydrogen atom contains one proton and one electron. There is no neutron in it.
Alkali Metals
Alkali metals are named thus because when they react with water they form compounds called alkalies (i.e. hydroxide compounds of these elements). For example, sodium hydroxide and potassium hydroxide.
- Spans from Lithium (Li) to Francium (Fr).
- Alkali metal is a group of six elements are in the Periodic Table.
- Shiny and soft. We can easily them with a knife.
- Highly reactive.
- Stored in oil to prevent reaction with air.
- Burst into flames when come in contact with water.
- Do not exist in free form. Always found in salts.
- Alkali metals are Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr).
- Among alkali metals, Sodium is the most abundant.
Alkaline-earth metals
When compounds of alkaline-earth metals are mixed in solutions, the solution become basic or alkaline (having pH higher than 7). That’s why the name alkaline.
- Span from Beryllium (Be) through Radium (Ra).
- Alkaline-earth metal is a group of six elements are in the Periodic Table.
- Shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.
- Alkaline-earth metals are Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra).
- All the alkaline-earth metals exist in nature except for Radium, which is comes into existence through decay of Uranium or Thorium.
- None of the alkaline earth metals are found in their elemental state.
Transition Metal
A transition metal is “an element whose atom has a partially filled d sub-shell, or which can give rise to cations with an incomplete d sub-shell”.
- Span from Titanium (Ti) through Copernicium (Cn).
- Hard but malleable. Have luster.
- Much less reactive than alkaline-earth metals.
- Good conductors of heat and electricity.
- Possess a high density and high melting points and boiling points
- All transition metals occur in solid state. Mercury is the only metal that exists in liquid state.
- Transition metal group consists of 38 elements in the Periodic Table.
- Transition metals are: Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc, Yttrium, Zirconium, Niobium, Molybdenum, Technetium, Ruthenium, Rhodium, Palladium, Silver, Cadmium, Hafnium, Tantalum, Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold, Mercury, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, , Darmstadtium, Roentgenium, and Copernicium.
Post-transition Metals
- These element are are soft / brittle and have poor mechanical strength.
- Melting points are lower than those of the transition metals.
- Post-transition metal group includes Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl), Tin (Sn), Lead (Pb), and Bismuth (Bi).
Metalloids
- These elements have properties in between those of metals and nonmetals.
- Metalloids are also called “semimetals” or “poor metals.”
- They have a metallic appearance, but they are brittle.
- Unlike metals, which are good conductors, metalloids are semiconductors of electricity. For example, Silicon (Si) and Germanium (Ge).
- Chemically, they behave mostly as nonmetals.
- Metalloids can form alloys with metals.
- Metalloid group includes Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), and Polonium (Po).
Nonmetals
- These elements lack metallic properties.
- When in solid state, they are brittle.
- Poor conductors of electricity and heat.
- Sometimes, metalloids are also counted as nonmetals.
- Nonmetal group includes Hydrogen (H), Helium (He), Carbon (C), Nitrogen (N), Phosphorus (P), Oxygen (O), Sulfur (S), and Selenium (Se).
- Just two of the nonmetals, hydrogen and helium, make 99% of the universe.
Halogens
- Halogen is the only group in Periodic Table which has elements that exists in solid, liquid as well as gaseous state.
- Fluorine and Chlorine exist as gases at room temperature; Bromine as liquid and Iodine and Astatine exist as solids.
- The word halogen means salt-producing. When halogen elements react with metals, they produce salts.
- Halogen group contains five elements: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At).
Nobel Gases
- Nobel gases group contain six elements: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn), and Oganesson (Og).
- These gases are all odorless, colorless gases.
- Nobel gases posses very low chemical reactivity. That is why they are also called inert gases.
- These are very suitable in applications where reactions are not wanted.
Lanthanides
- Lanthanides group contains 15 elements in the Periodic Table.
- These 15 elements are: Lanthanum, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, and Lutetium.
- Also known as rare earth elements.
Actinides
- This group span from Actinium (Ac) through Lawrencium (Lr)
- Actinide group of Periodic Table contains 15 elements: Actinium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium, Mendelevium, Nobelium, and Lawrencium.
- All actinides are radioactive.
- Only Uranium and Thorium exist naturally.
- Uranium, Thorium and synthesized Plutonium are the most abundant actinides on Earth.
Use the citation below to add this article to your bibliography
"Periodic Table Groups: Names and Properties." Dashamlav.com. Web. 22 October 2024. <https://dashamlav.com/periodic-table-groups-names-properties/>
Dashamlav.com, "Periodic Table Groups: Names and Properties." Accessed 22 October 2024. https://dashamlav.com/periodic-table-groups-names-properties/
"Periodic Table Groups: Names and Properties." (n.d.). Dashamlav.com. Retrieved 22 October 2024 from https://dashamlav.com/periodic-table-groups-names-properties/
